Development of Borate-Based Basic Ionic Liquid for Room Temperature CO2
Capture
ACS Omega, 2025, 10, 46, 56782–56794
Jun Hang Chia and Takuya Harada
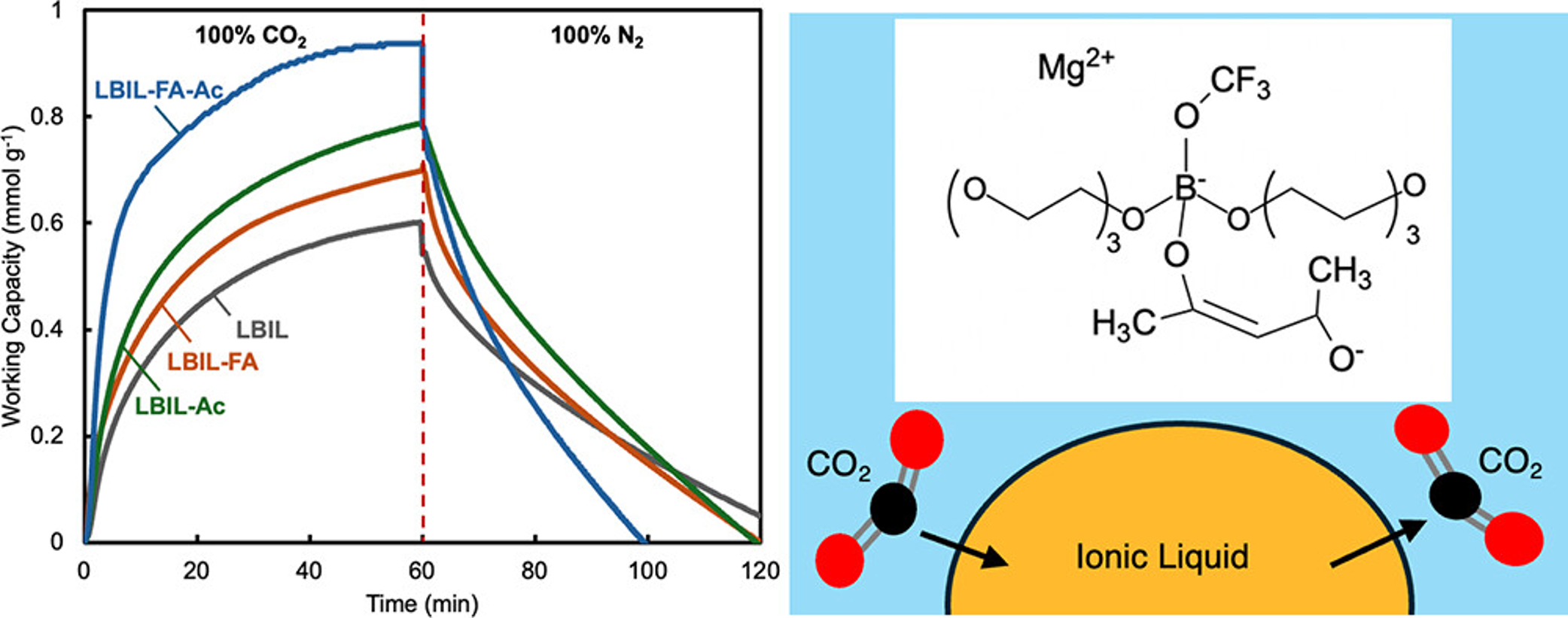
As part of CO2 capture strategies, ionic liquid-based CO2 absorbents have
gained attention for their tunable properties to lower the energy costs
for CO2 capture. In this study, a series of borate-based nonamine functionalized
ionic liquids (ILs), incorporated with magnesium acetylacetonate, were
developed and investigated for its CO2 capture capability at moderate temperature
under ambient pressure. Nuclear magnetic resonance and Fourier transform
infrared spectroscopy confirmed the successful incorporation of acetylacetonate
ligands into the fluorinated-lithium borate ionic liquids. Comprehensive
analyses of the physical and thermochemical properties revealed that the
synthesized ILs remain stable below 200 °C, with the borate structure and
acetylacetonate ligands intact. The ILs functionalized with fluorinated
alcohol and magnesium acetylacetonate enhance the CO2 uptake capacity by
55% in comparison with the original lithium borate ILs, suggesting the
enhanced cooperative interactions responsible for improved CO2 capture
performance. The carbon capture mechanism was identified to proceed via
physical absorption, as evidenced by minimal changes in the characterization
results and viscosity after CO2 absorption. The enthalpy of CO2 absorption
(ΔHa) for the synthesized ILs were determined experimentally by using differential
scanning calorimetry to be in the range from −12.4 kJ mol–1 to −18.9 kJ
mol–1, which are much lower than that of conventional amine solutions (e.g.,
MEA: −82 kJ mol–1) and amine-based ILs ((e.g., [Bmim][Ac]: −45.8 kJ mol–1).
These findings suggest that lithium borate-acetylacetonate ILs offer a
promising approach for a CO2 capture system under ambient conditions.
CO2-Promoted Assembly of Nonplanar B12O24-Ring as a Fundamental Building
Block in Lithium–Sodium Alkali Borates during Carbon Capture
Inorganic Chemistry, 2025, 64,17,8678-8684
David Unnervik and Takuya Harada

The crystalline structure of Li3NaB4O8, a reaction product resulting from CO2 absorption by lithium–sodium orthoborate ((Li0.5Na0.5)3BO3), a recently developed sorbent for high-temperature carbon capture, is herein elucidated. The compound crystallizes to form a peculiar isolate borate fundamental building block consisting of six 3-membered borate rings interlinked through mutual tetrahedral borates to form a nonplanar 6-membered borate ring of chemical formula B12O24. The identification of this structure allows closing of the loop regarding the reaction mechanism characterizing CO2 capture by lithium–sodium orthoborate, sheds light on previously made observations regarding the evolution of the physicochemical properties of the melt during carbon capture, and provides valuable information for future studies and process simulations involving this promising new material for carbon capture.
Advancing Molten Alkali Borate Sorbents for High-Temperature Carbon Capture
by Structural Elucidation of the Ionic-Oxide Reaction Mechanism
Advanced Sustainable Systems, 2025, 2400969 (https://doi.org/10.1002/adsu.202400969)
David Unnervik and Takuya Harada

The structural complexity of alkali borates, evident in the wide range of distinct structures that typically comprise these compounds, is responsible for the significant differences observed in the physicochemical properties of their corresponding melts. In this work, the structural transformations arising from carbon capture using molten lithium-sodium orthoborate ((Li0.5Na0.5)3BO3), a promising new alkali borate sorbent for carbon capture, are investigated to better understand the evolution of various physicochemical properties of the melt by employing in situ high-temperature Fourier transform infrared spectroscopy in conjunction with density functional theory. The carbon capture mechanism is shown to proceed via polymerization of small orthoborate segments (BO33-) into larger structural units, ultimately reaching the metaborate composition (BO2-) in the form of Li3NaB4O8 and Na3B3O6 upon complete CO2 saturation. The introduction of carbonate ions in the non-polymerized orthoborate melt via CO2-capture has a substantial diluting effect on the density and viscosity of the resulting polymerized CO2-saturated melt. Investigations into the melting points associated with the various compounds involved in the capture mechanism and the discovery of an apparent second-order phase transition of lithium-sodium pyroborate (Li2Na2B2O5) past 520 ℃ further provide new and valuable information as to potential energetically favorable operating conditions for this absorber/regenerator-based carbon capture technology.

Alkali metal borate molten salts have emerged as efficient high-temperature
liquid CO2 sorbents, advancing carbon reduction for energy-intensive industrial
chemical processes. This work investigated the relationship between the
liquidus behavior and CO2 uptake characteristics of lithium–sodium borates,
MxB1–xO1.5–x (M = Li0.5Na0.5), over a composition range of 0.50 ≤ x ≤ 0.80.
Differential Scanning Calorimetry (DSC) measurements revealed detailed
phase–transition profiles, with liquidus temperatures ranging from 500
to 650 °C. Composition-dependent liquidus behavior governs the CO2 sorption
characteristics during the early sorption stages, transitioning from “solid-to-liquid”
in low-alkali to “liquid-to-liquid” in high-alkali regions. Optimal working
capacities and reaction rates consistently correspond to the liquidus transition
range, minimizing energy demands for preserving molten state in the cyclic
CO2 capture-release operations. These findings establish temperature–composition
operating windows tailored to industrial needs, providing critical liquidus
diagrams and demonstrating their potential as versatile sorbents for high-temperature
CO2 capture.
Importance of the Oxide-Ion Activity on the Hot Corrosion of Nickel at
the Interface with Molten Borate-Based Sorbents During CO2 Capture
The Journal of Physical Chemistry C, 2024, 128, 39, 16813-16823
David Unnervik and Takuya Harada
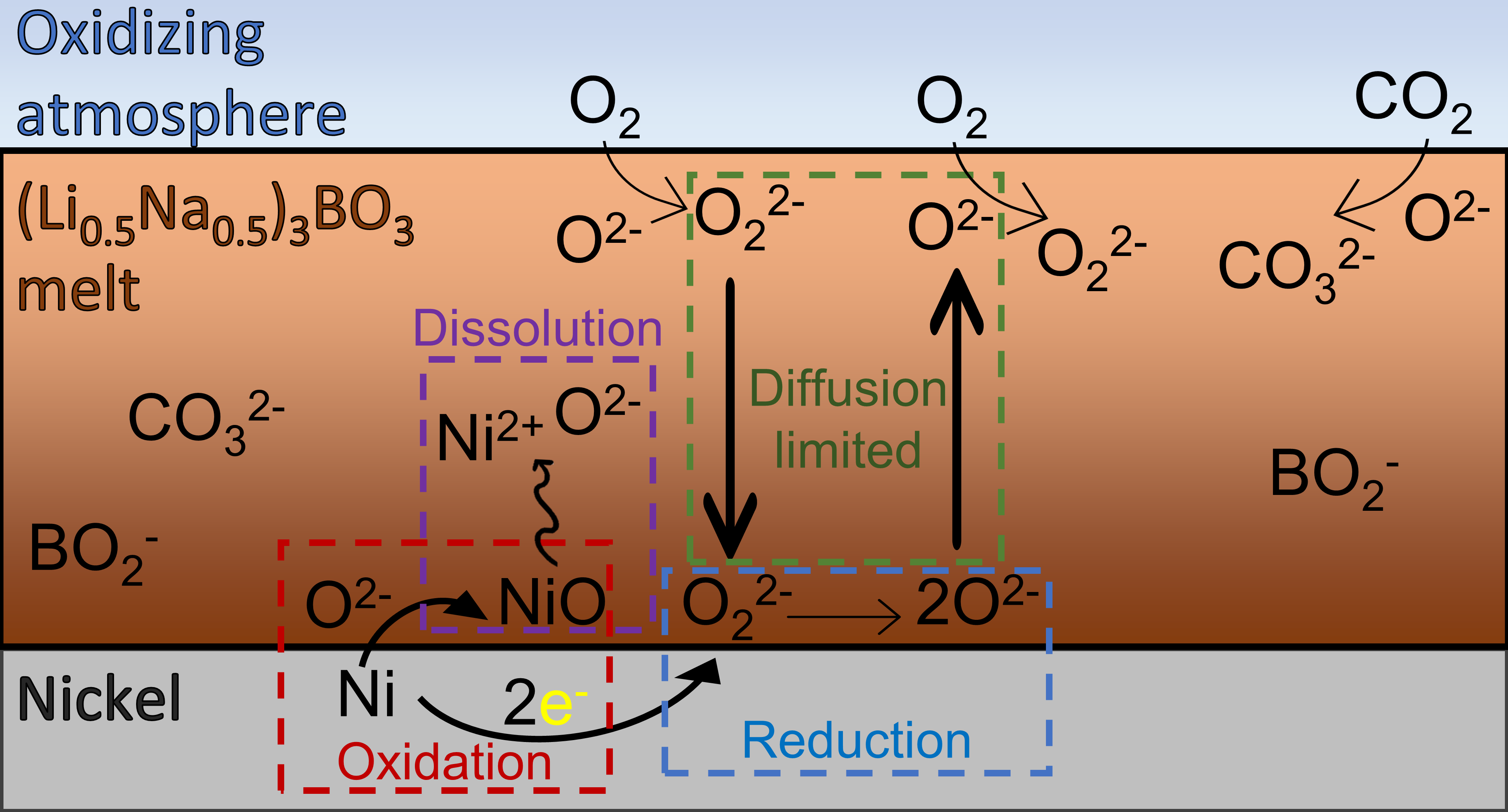
The oxidation and subsequent dissolution of nickel was investigated in molten lithium–sodium orthoborate ((Li0.5Na0.5)3BO3) to assess the scaling-up potential of this modern sorbent for carbon capture through its compatibility with typical reactor materials used in processes involving molten salts. Results indicate the oxidation process to be diffusion-limited and to proceed through the chemical dissolution of oxygen in the melt via reaction with oxide ions, showcasing the decisive role of the oxide-ion activity on the oxidation rate. Additionally, an observed loss in carbon capture capacity over time is explained by the reaction of the sorbent with nickel oxide, thus encouraging reactor designs in which the ratio between the metal/melt interfacial area and the bulk volume is minimized. The dissolution of nickel appears to partially follow an acidic regime, with the upper solubility limits approaching 1000 ppm. These results mirror previously reported mechanisms on lithium–sodium carbonate and shed further light on the scale-up potential of this innovative class of sorbents for carbon capture.
High-throughput screening of nano-hybrid metal–organic-frameworks for photocatalytic
CO2 reduction
Materials Horizons, 2024, 11, 18, 4311-4320
Moin Khwaja and Takuya Harada

Photocatalytic conversion of CO2 into fuel feed stocks is a promising method for sustainable fuel production. A highly attractive class of materials, inorganic-core@metal–organic-framework heterogeneous catalysts, boasts a significant increase in catalytic performance when compared to the individual materials. However, due to the ever-expanding chemical space of inorganic-core catalysts and metal–organic frameworks (MOFs), identification of these optimal heterojunctions is difficult without appropriate computational screening. In this work, a novel high-throughput screening method of nano-hybrid photocatalysts is presented by screening 65 784 inorganic-core materials and 20 375 MOF-shells for their ability to reduce CO2 based on their synthesizability, aqueous stability, visible light absorption, and electronic structure; the passing materials were then paired based on their electronic structure to create novel heterojunctions. The results showed 58 suitable inorganic-core materials and 204 suitable MOFs ranging from never-before-synthesized catalysts to catalysts that have been overlooked for their photocatalytic ability. These materials lay a new foundation of photocatalysts that have passed theoretical requirements and can significantly increase the rate of catalyst discovery.
Wetting Kinetics of Lithium–Sodium Orthoborate on Nickel and Stainless
Steel under CO2-Lean and Saturated Conditions
Langmuir, 2024 , 40, 15, 8059-8066
David Unnervik and Takuya Harada

Contact angle measurements were conducted on lithium–sodium orthoborate,
an alkali-metal borate-based high-temperature carbon capture sorbent, in
contact with nickel and stainless steel under air, N2, and CO2 atmospheres
at 600 °C in order to assess the compatibility of this innovative sorbent
with packed-bed reactors, typically used in the commercial carbon capture
industry. On nickel, the results reveal the melt to be wetting (contact
angle θ ≤ 90°), albeit with a contact angle reaching an apparent equilibrium
over the experimental time considered. It was found that θ was lower under
N2 than under CO2, which was explained as a consequence of the oxide-ion
activity difference between the CO2-lean and saturated melts. On stainless
steel, however, the melt rapidly spreads, tending toward complete wetting
of the metal sheet (θ = 0°) in all atmospheres. For the two metals investigated,
the results indicate high to very high wettability, hence the adequacy
of using lithium–sodium orthoborate in conventional gas–liquid contactors,
further backing this sorbent as a potential alternative to existing sorbents
for CO2 capture.
Carbonate-Induced Structural Transformation of Lithium-Sodium Orthoborate
to a Low-Viscosity Metaborate via CO2Capture
ACS Applied Materials & Interfaces, 2023, 15, 54667-54676
David Unnervik and Takuya Harada

This paper reports on the structural changes occurring within the lithium–sodium
orthoborate crystal lattice during the solid-state absorption of CO2. Results
derived from Fourier transform infrared measurements indicate that the
CO2-saturated mixed-alkali metal orthoborate and its CO2-lean metaborate
counterpart essentially present the same spectral profile, suggesting that
CO2 capture results in a fundamental shift of the orthoborate composition
to the metaborate one. The implications of such a structural transformation
were examined in the molten state at elevated temperatures through rheological
measurements, and although confirming that the CO2-lean metaborate exhibits
a higher viscosity than the CO2-lean orthoborate, the results suggest that
incorporation of CO2 in the orthoborate ionic lattice dilutes the melt,
leading to a remarkable reduction in its overall viscosity, despite causing
a structural transformation from the less viscous orthoborate form to the
more viscous metaborate one.
Molten ionic oxides for CO2capture at medium to high temperatures
Journal of Materials Chemistry A, 2019, 7, 21827-21834
Takuya Harada, Cameron Halliday, Aqil Jamal, and T. Alan Hatton
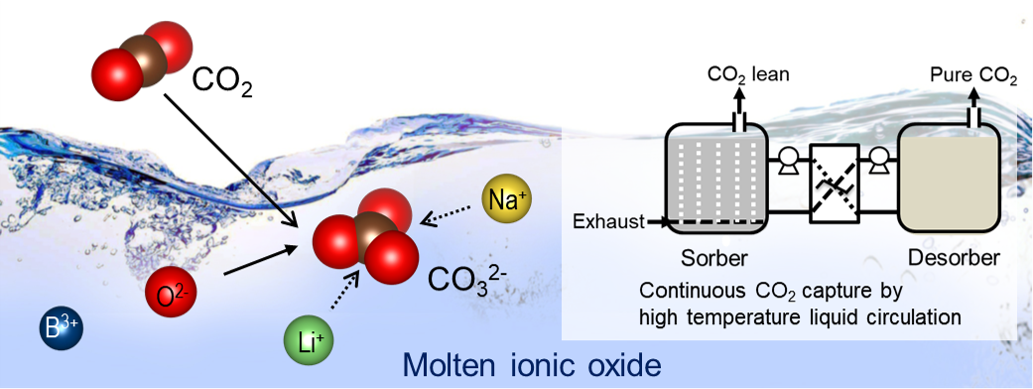
The development of efficient low cost CO2 capture systems is a critical challenge for mitigating climate change while meeting global energy demand. Herein, we demonstrate the first liquid absorbents for CO2 capture at medium to high temperatures (500 to 700 °C). Molten ionic oxides based on sodium borate and the mixed alkali-metal borates show remarkably fast kinetics and intrinsic regenerability, with no observable deterioration in performance over multiple absorption–desorption cycles under both temperature- and pressure-swing operations. The behavior of the molten ionic oxides is ascribed to the instantaneous formation of carbonate ions in the molten oxides without the diffusional transport restrictions imposed by solid product layers characteristic of solid adsorbents. The new liquid absorbents will enable continuous processing and thermal integration via a simple absorber–desorber arrangement, thereby overcoming the challenges previously restraining high temperature CO2 capture and opening up new opportunities in clean energy production.
Nonvolatile Colloidal Dispersion of MgO Nanoparticles in Molten Salts for
Continuous CO2Capture at Intermediate Temperatures
ACS Sustainable Chemistry and Engineering, 2019, 7, 7979-7986
Takuya Harada, Paul Brown, and T. Alan Hatton

The establishment of advanced CO2 capture, utilization, and storage (CCUS) technology is a crucial challenge for the mitigation of serious ongoing climate change. Herein, we report nonaqueous colloidal dispersions of MgO nanoparticles in molten salts as a new class of fluid absorbents for continuous CO2 capture at intermediate temperatures ranging from 200 to 350 °C. The colloidal absorbents were developed by dispersion of the nanoparticles in three different types of thermally stable low-melting point salts: ternary-eutectic alkali-metal nitrates ((Li–Na–K)NO3), tetraphenylphosphonium bis(trifluoromethane)sulfonimide ([P(Ph)4][NTf2]), and their mixtures. The new absorbents show high CO2 uptake performance with acceptable rheological properties at the target temperatures. The analysis of reaction rate kinetics in the uptake of CO2 revealed that CO2 can diffuse quickly into the molten salts to initiate the rapid formation of carbonates on the surfaces of MgO nanoparticles dispersed in these molten salts. These results demonstrate that the new colloidal dispersions could be used as fluid absorbents for advanced continuous CO2capture processes at the temperatures of exhausts from fossil fuel combustion reactors without the energy losses incurred upon cooling of the gases as required for traditional absorption systems.
Tri-lithium borate (Li3BO3); a new highly regenerable high capacity CO2adsorbent at intermediate temperature
Journal of Materials Chemistry A, 2017, 5, 22224-22233
Takuya Harada, T. Alan Hatton
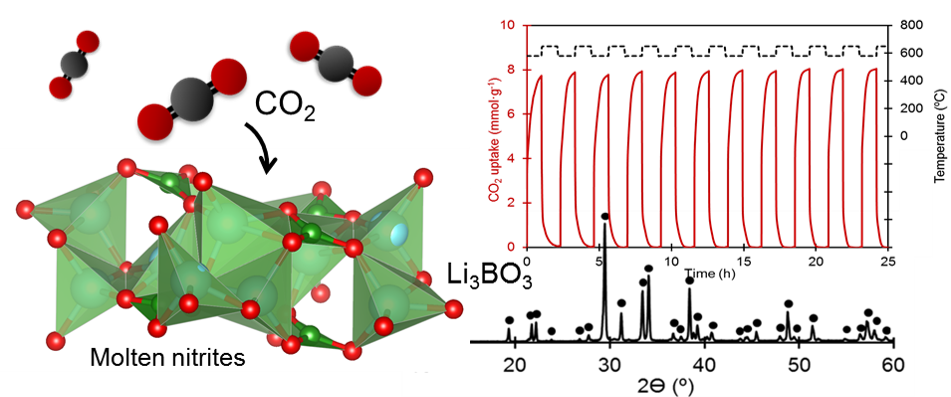
A lithium-borate oxide, Li3BO3, is proposed as a next generation high capacity CO2 adsorbent operative over the intermediate temperature range of 500 to 650 °C. This adsorbent shows high CO2 uptake capacity (e.g., 11.3 mmol g−1 at 520 °C) with excellent cyclic regenerability in the presence of alkali-metal nitrite salts as a reaction facilitator. The high CO2 uptake is attributed to the dissociative formation of lithium carbonate (Li2CO3) and different compositions of lithium borates (Li6B4O9, LiBO2 and Li2B4O7) during the reaction of Li3BO3 with CO2. The excellent performance of the new CO2 adsorbents is discussed in terms of rapid gas–solid reactions on Li3BO3 mediated by the molten nitrite salts and pinning effects that prevent the sintering of particle grains.
Alkali Metal Nitrate-Promoted High-Capacity MgO Adsorbents for Regenerable
CO2Capture at Moderate Temperatures
(*This work was press released as ACS News by American Chemical Society, and featured in Phys. Org. Science Daily, and others.)
Chemistry of Materials, 2015, 27, 1943-1949
Takuya Harada, Fritz Simeon, Esam Z. Hamad, and T. Alan Hatton
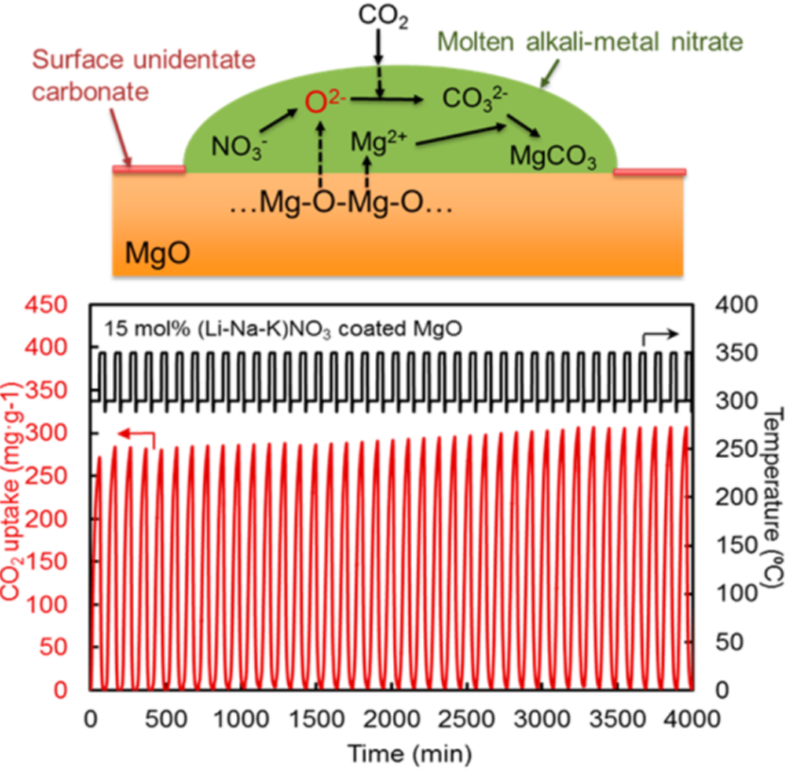
Regenerable high capacity CO2sorbents are desirable for the establishment of widespread carbon capture and storage (CCS) systems to reduce global CO2emissions. We report on the marked effects of molten alkali metal nitrates on CO2uptake by MgO particles and their impact on the development of highly regenerable CO2adsorbents with high capacity (>10.2 mmol g–1) at moderate temperatures (∼300 °C) under ambient pressure. The molten alkali metal nitrates are shown to prevent the formation of a rigid, CO2-impermeable, unidentate carbonate layer on the surfaces of MgO particles and promote the rapid generation of carbonate ions to allow the high rate of CO2uptake.
Formation of magnetic nanotubes by the cooperative self-assembly of chiral
amphiphilic molecules and Fe3O4nanoparticles
Physical Chemistry Chemical Physics (PCCP), 2010, 12, 11938-11943
Takuya Harada, Fritz Simeon, John B. Vander Sande, and T. Alan Hatton

Single- and double-walled magnetic nanotubes are obtained in a one-step
liquid phase reaction by the cooperative self-assembly of chiral amphiphiles
and nanoparticles on cooling of heated mixtures of N-dodecanoyl-L-serine
and Fe3O4 nanoparticles in toluene. The nanotubes are composed of well-ordered,
close-packed nanoparticle assemblies, and can be transformed into chiral
magnetic nanostructures, such as helical coils, by subsequent calcination.
The nanoparticle assemblies and their variations on calcination are attributed
to the collective organization of the surfactant molecules adsorbed on
the nanoparticles and the freely dispersed chiral molecules, and the dewetting
effects guided by the primitive constitution of the chiral amphiphilic
molecular assemblies.
Formation of Highly Ordered Rectangular Nanoparticle Superlattices by the
Cooperative Self-Assembly of Nanoparticles and Fatty Molecules.
Langmuir, 2009, 25, 6407-6412
Takuya Harada, T. Alan Hatton
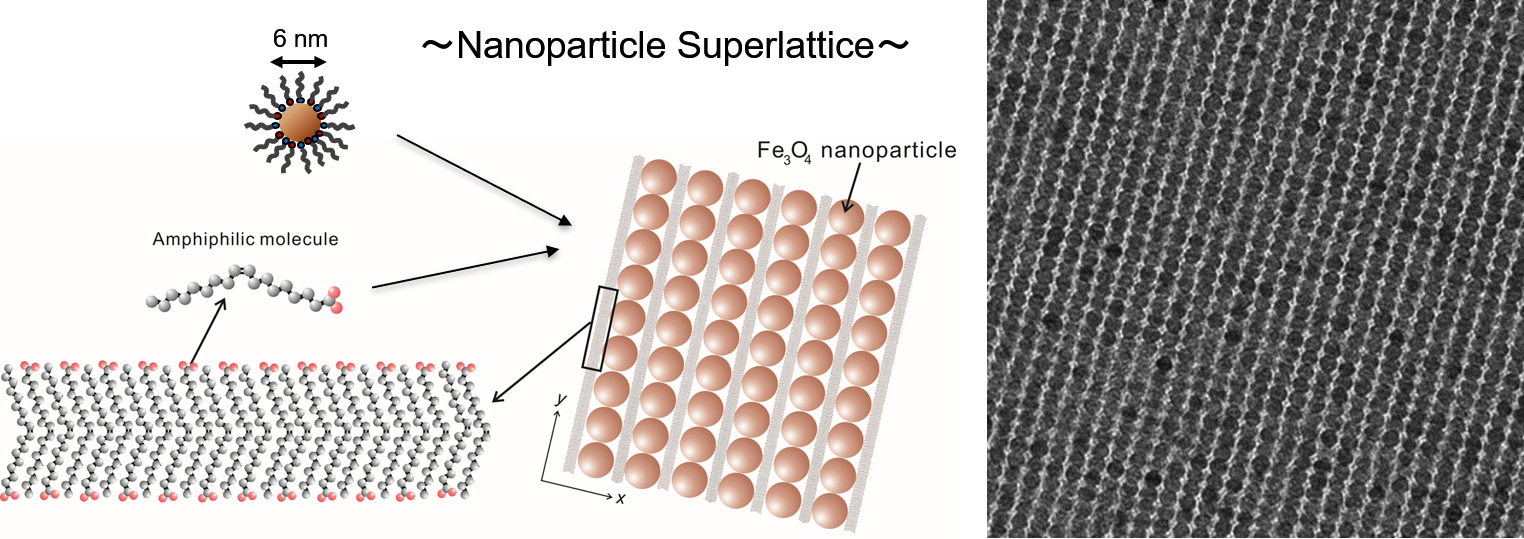
We demonstrate the formation of highly ordered twofold symmetric rectangular nanoparticle superlattices by the slow evaporation of solvent from colloidal dispersions of oleic acid/oleylamine-coated Fe3O4 nanoparticles on a water surface. These superlattices covered regions of micrometers in size without any noticeable disorders or defects, with size controlled by the amount of oleic acid added to the colloidal dispersions. The superlattices were transformed into arrays of nanowires by subsequent calcination. The peculiar nanoparticle assemblies are discussed in terms of the cooperative self-assembly of nanoparticles and fatty molecules during the slow evaporation of solvent.