中高温CO2吸収剤の液体化に初めて成功!連続CO2回収プロセスの実用化を大きく前進!!
Molten ionic oxides for CO2capture at medium to high temperatures
Journal of Materials Chemistry A, 2019, 7, 21827-21834
Takuya Harada, Cameron Halliday, Aqil Jamal, and T. Alan Hatton
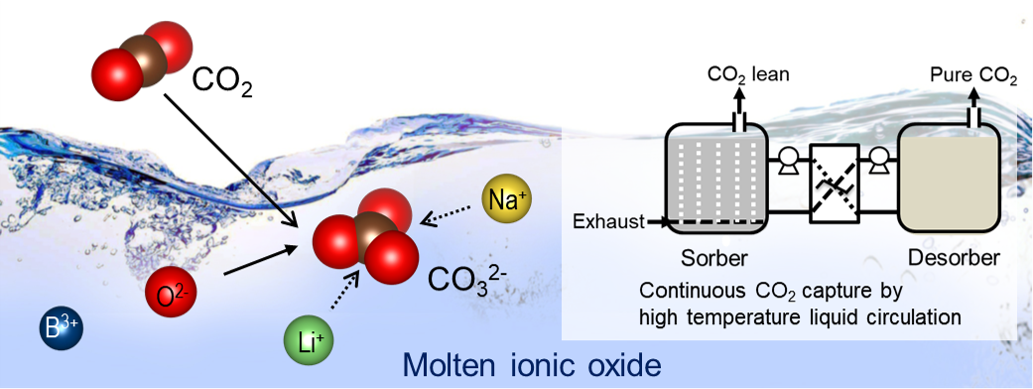
The development of efficient low cost CO2 capture systems is a critical challenge for mitigating climate change while meeting global energy demand. Herein, we demonstrate the first liquid absorbents for CO2 capture at medium to high temperatures (500 to 700 °C). Molten ionic oxides based on sodium borate and the mixed alkali-metal borates show remarkably fast kinetics and intrinsic regenerability, with no observable deterioration in performance over multiple absorption–desorption cycles under both temperature- and pressure-swing operations. The behavior of the molten ionic oxides is ascribed to the instantaneous formation of carbonate ions in the molten oxides without the diffusional transport restrictions imposed by solid product layers characteristic of solid adsorbents. The new liquid absorbents will enable continuous processing and thermal integration via a simple absorber–desorber arrangement, thereby overcoming the challenges previously restraining high temperature CO2 capture and opening up new opportunities in clean energy production.
ナノ粒子コロイドCO2吸収剤の開発!
Nonvolatile Colloidal Dispersion of MgO Nanoparticles in Molten Salts for
Continuous CO2Capture at Intermediate Temperatures
ACS Sustainable Chemistry and Engineering, 2019, 7, 7979-7986
Takuya Harada, Paul Brown, and T. Alan Hatton
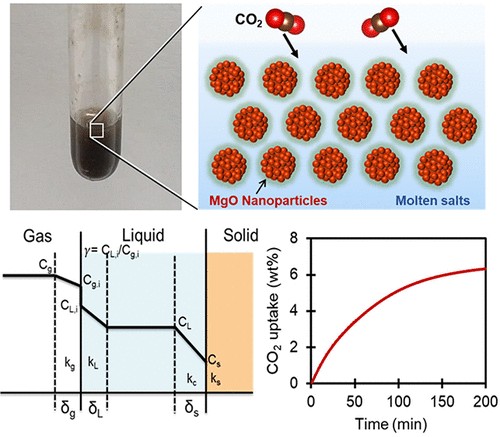
The establishment of advanced CO2 capture, utilization, and storage (CCUS) technology is a crucial challenge for the mitigation of serious ongoing climate change. Herein, we report nonaqueous colloidal dispersions of MgO nanoparticles in molten salts as a new class of fluid absorbents for continuous CO2 capture at intermediate temperatures ranging from 200 to 350 °C. The colloidal absorbents were developed by dispersion of the nanoparticles in three different types of thermally stable low-melting point salts: ternary-eutectic alkali-metal nitrates ((Li–Na–K)NO3), tetraphenylphosphonium bis(trifluoromethane)sulfonimide ([P(Ph)4][NTf2]), and their mixtures. The new absorbents show high CO2 uptake performance with acceptable rheological properties at the target temperatures. The analysis of reaction rate kinetics in the uptake of CO2 revealed that CO2 can diffuse quickly into the molten salts to initiate the rapid formation of carbonates on the surfaces of MgO nanoparticles dispersed in these molten salts. These results demonstrate that the new colloidal dispersions could be used as fluid absorbents for advanced continuous CO2capture processes at the temperatures of exhausts from fossil fuel combustion reactors without the energy losses incurred upon cooling of the gases as required for traditional absorption systems.
アルカリ金属ホウ酸塩の大容量・高サイクル特性を確認!
Tri-lithium borate (Li3BO3); a new highly regenerable high capacity CO2adsorbent at intermediate temperature
Journal of Materials Chemistry A, 2017, 5, 22224-22233
Takuya Harada, T. Alan Hatton
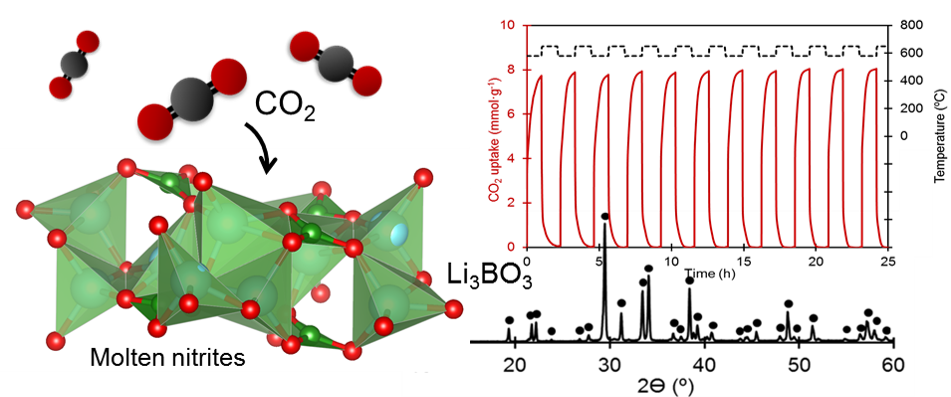
A lithium-borate oxide, Li3BO3, is proposed as a next generation high capacity CO2 adsorbent operative over the intermediate temperature range of 500 to
650 °C. This adsorbent shows high CO2 uptake capacity (e.g., 11.3 mmol g−1 at 520 °C) with excellent cyclic
regenerability in the presence of alkali-metal nitrite salts as a reaction
facilitator. The high CO2 uptake is attributed to the dissociative formation of lithium carbonate
(Li2CO3) and different compositions of lithium borates (Li6B4O9, LiBO2 and Li2B4O7) during the reaction of Li3BO3 with CO2. The excellent performance of the new CO2 adsorbents is discussed in terms of rapid gas–solid reactions on Li3BO3 mediated by the molten nitrite salts and pinning effects that prevent
the sintering of particle grains.
溶融イオン酸化物によるMgOのCO2反応促進メカニズムの解明!
(*本研究内容は、ACS Newsとしてアメリカ化学会からプレスリリースされました 。)
Alkali Metal Nitrate-Promoted High-Capacity MgO Adsorbents for Regenerable
CO2Capture at Moderate Temperatures
Chemistry of Materials, 2015, 27, 1943-1949
Takuya Harada, Fritz Simeon, Esam Z. Hamad, and T. Alan Hatton
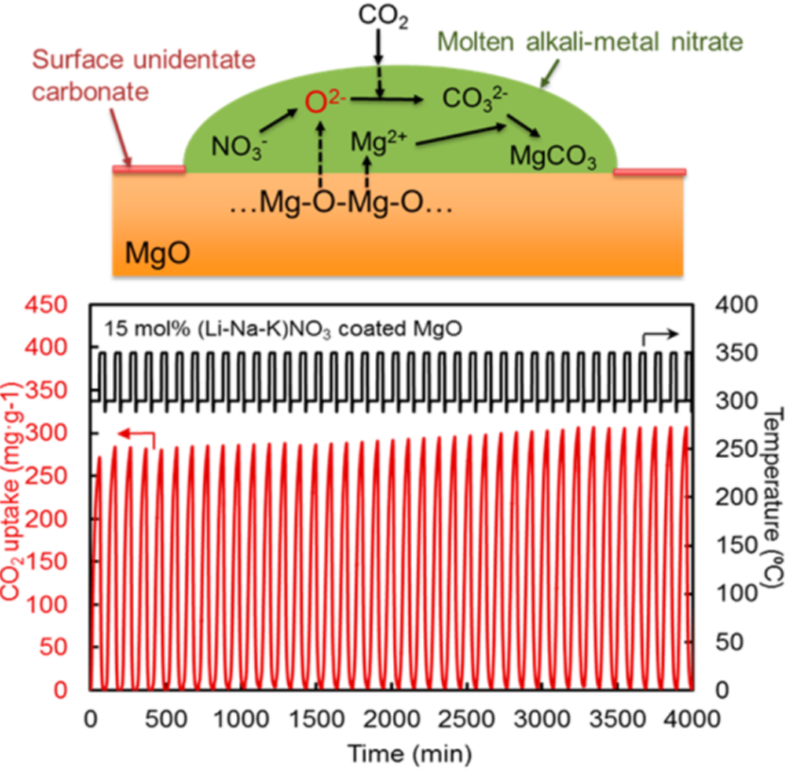
Regenerable high capacity CO2sorbents are desirable for the establishment of widespread carbon capture
and storage (CCS) systems to reduce global CO2emissions. We report on the marked effects of molten alkali metal nitrates
on CO2uptake by MgO particles and their impact on the development of highly regenerable
CO2adsorbents with high capacity (>10.2 mmol g–1) at moderate temperatures (∼300 °C) under ambient pressure. The molten
alkali metal nitrates are shown to prevent the formation of a rigid, CO2-impermeable, unidentate carbonate layer on the surfaces of MgO particles
and promote the rapid generation of carbonate ions to allow the high rate
of CO2uptake.
キラル両親媒分子とナノ粒子の協奏効果による機能性ナノ粒子ナノチューブの形成!
Formation of magnetic nanotubes by the cooperative self-assembly of chiral
amphiphilic molecules and Fe3O4nanoparticles
Physical Chemistry Chemical Physics (PCCP), 2010, 12, 11938-11943
Takuya Harada, Fritz Simeon, John B. Vander Sande, and T. Alan Hatton

Single- and double-walled magnetic nanotubes are obtained in a one-step
liquid phase reaction by the cooperative self-assembly of chiral amphiphiles
and nanoparticles on cooling of heated mixtures of N-dodecanoyl-L-serine
and Fe3O4 nanoparticles in toluene. The nanotubes are composed of well-ordered,
close-packed nanoparticle assemblies, and can be transformed into chiral
magnetic nanostructures, such as helical coils, by subsequent calcination.
The nanoparticle assemblies and their variations on calcination are attributed
to the collective organization of the surfactant molecules adsorbed on
the nanoparticles and the freely dispersed chiral molecules, and the dewetting
effects guided by the primitive constitution of the chiral amphiphilic
molecular assemblies.
有機-無機協奏的自己組織化による大規模ナノ粒子超格子の形成!
Formation of Highly Ordered Rectangular Nanoparticle Superlattices by the
Cooperative Self-Assembly of Nanoparticles and Fatty Molecules.
Langmuir, 2009, 25, 6407-6412
Takuya Harada, T. Alan Hatton
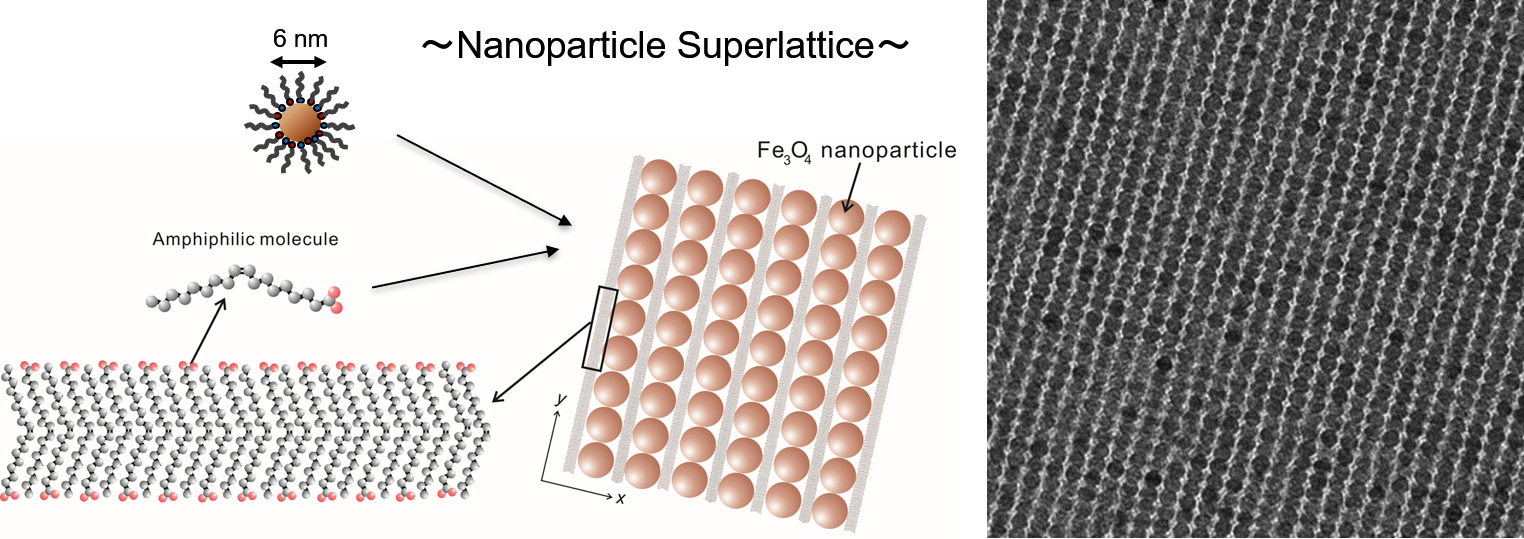
We demonstrate the formation of highly ordered twofold symmetric rectangular nanoparticle superlattices by the slow evaporation of solvent from colloidal dispersions of oleic acid/oleylamine-coated Fe3O4 nanoparticles on a water surface. These superlattices covered regions of micrometers in size without any noticeable disorders or defects, with size controlled by the amount of oleic acid added to the colloidal dispersions. The superlattices were transformed into arrays of nanowires by subsequent calcination. The peculiar nanoparticle assemblies are discussed in terms of the cooperative self-assembly of nanoparticles and fatty molecules during the slow evaporation of solvent.